Biosensors
Electrochemical sensors used for biosensing applications are very relevant nowadays to easily analyze concentrations of chemical species, specifically compounds that could possibly exist within biological samples. Hydrogen peroxide (H2O2) is an important intermediate in biological processes and is also widely used in food and cosmetic industry as redox active reagent. Our group focuses on developing different materials that can serve as highly sensitive electrochemical sensors for detection and quantification of H2O2.
Metal Oxide-Based Biosensors
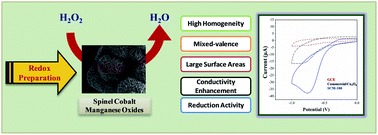
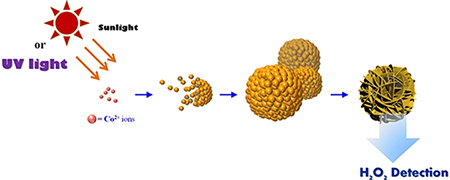
First row transition metal oxides are quite interesting towards their electrocatalytic properties which is more affordable to fabricate than noble metals. Our group utilized the redox properties of permanganate (MnO4-) to allow oxidation of other metals forming a mixed metal oxide for such applications. The redox couple between the two metals (Co, Mn, and Cr) has allowed to selectively detect H2O2 under physiological pH conditions with a detection limit of 15 μM. [1] The as-produced cobalt manganese oxide was further hybridized with graphene to exhibit much higher sensitivity, presumably due to the formation of new active sites and improved charge transport. Cobalt oxide-based H2O2 sensors through a sunlight-catalyzed redox process under aqueous environment has been successfully revealed. The resulting nanomaterial produced a wide H2O2 detection range (0.005 to 35 mM) with high selectivity and a lower detection limit (0.7 μM) compared to utilizing permanganate as an oxidizing agent. [2]
Heterojunction Interface for H2O2 sensing
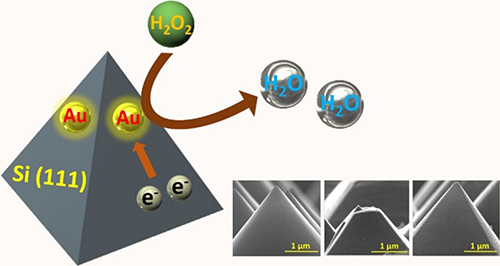
Utilizing high-index surfaces has attracted a lot of interest as it yields better activity towards different catalytic reactions. Our research explored on the deposition of Au onto facet-specific pyramidal Si (111). The exposed interface between Au and Si (111) has been proven to have an enhanced sensitivity than other faceted Au/Si catalysts, showing that the heterojunction between the two materials are critical in enhancing electrochemical sensing activity.
References/Related Works:
[1] Kuo, C.-C.; Lan, W.-J.; Chen, C.-H.* Redox preparation of mixed-valence cobalt manganese oxide nanostructured materials: highly efficient noble metal-free electrocatalysts for sensing hydrogen peroxide. Nanoscale 6, 334 (2014)
[2] Su, C.-Y.; Lan, W.-J.; Chu, C.-Y.; Liu, X.-J.; Kao, W.-Y.; Chen, C.-H.* Photochemical Green Synthesis of Nanostructured Cobalt Oxides as Hydrogen Peroxide Redox for Bifunctional Sensing Application. Electrochim. Acta 190, 588 (2016)
[3] Huang, C.-W.; Valinton, J. A. A.; Hung, Y.-J.; Chen, C.-H.* Facet-specific Heterojunction in Gold-decorated Pyramidal Silicon for Electrochemical Hydrogen Peroxide Sensing. Sens. Actuators B: Chem. 226, 463 (2018)
[4] Lan, W.-J.; Chen, C.-H.* Hybridization of Graphene in 3D Complex Nanovoids: Synergistic Nanocomposites for Electrocatalytic Reduction of Hydrogen Peroxide. Electrochim. Acta 180, 1014 (2015)
[5] Lan, W.-J.; Kuo, C.-C.; Chen, C.-H.* Hierarchical Nanostructures with Unique Y-shaped Interconnection Networks in Manganese Substituted Cobalt Oxides: The Enhancement Effect on Electrochemical Sensing Performance. Chem. Commun. 49, 3025 (2013)


